-
- Posts: 1
- Joined: Sun Nov 23, 2025 5:37 pm
We are working on a methodology for separation and quantitation of API and Specified Impurity. API is a pyrazolone family salt. Separation itself is no problem. Sample is injected at high concentration so we can also see and report unidentified impurities. We know API peak shape can not be the best because of this but our problem goes above that
Our main problem can be seen here:
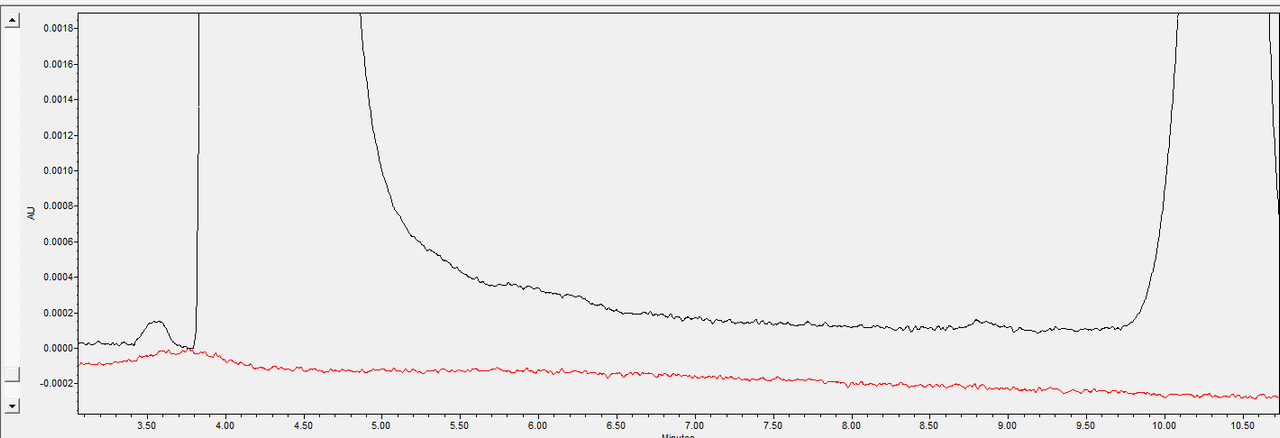
Black being sample, red being diluent. First big peak is obviously API. Second peak is our expected, specificified and identified degradation product. This "baseline" that does not finish to drop happens in all systems, all methodologies and operation conditions we have tried. Its very critical as we are unable to properly quantitate the impurity because of it. Injection volume itself does not help, unless we "hide" this "tail" by lowering sample concentration so much that unidentified impurities get lost.
Other problems range from fronting of API peak, tailing, split peak, unretained API peak (eluting before death volume time and diluent peak), among others.
And beside this, we have been experiencing very, very fast degradation and death of our columns. After around 50 to 80 injections, API peak shape starts looking bad, and just after a little while, chromatographic separation profile is completely lost. We suppose stationary phase is being attacked but as we have tried very different aquous mobile phases, only possibility is the API itself.
We have tried many methdologies/aplication notes/methodologies from publications, but after a few injections our problems remain. Ill try to briefly describe main operation conditions because they all differ from each other
Columns are C18 endcapped and certified for working at high pHs. Dimensions and brands differ. Tried 8 different columns to date.
Mobile phase has been different proportions of methanol (<50%) and different aquous buffers, ranging from pH 7.0 up to 11.0. Most of them are on the 7-8 pH region. Some have been phosphate buffers, others contain triethilamine, others require sodium hidroxide for pH adjustment.
Diluent for everyhing on the system is Methanol, because of said sample degradation (hydrolisis) with pure water.
Tried both HPLC and UPLC Systems.
Temperature is below 35°C on all systems
UV is 254 nm
API does not require sample preparation. Sample contains no contaminants, excipients, reagents, etc. Just API and diluent.
Sorry if my english was bad. I am not a native speaker.
Many thanks in advance for your help and suggestions. Regards!

